Basics of Nuclear Engineering:
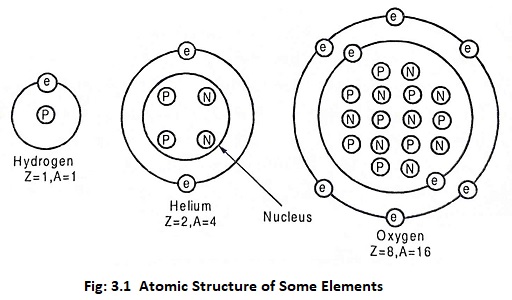
Basics of Nuclear Engineering – A matter is composed of atoms. An atom consists of a positive charged nucleus surrounded by a number of negatively charged electrons. The atomic structures of hydrogen, Helium and oxygen are shown in Fig. 3.1.
The nucleus contains protons and neutrons. The number of protons in a given atom is an atomic number (Z). So the atomic number for h is 1 and He is 2.
Some elements have the same number of protons in the nucleus but different number of neutrons. As a result, these elements have the same atomic number but different mass number. Such type of elements which have the same atomic number – the same number of protons -the same chemical properties but different mass numbers due to different number of neutrons, are known as the isotopes of an element.
The Uranium element has the same atomic number (92) but it has different isotopes with different mass number i.e. U233, U234, U235 , U236 and U238.
The isotopes having more than 80 are unstable. The nuclei of these isotopes are unstable and radio active and they decay by emitting electromagnetic radiations in the form of α – particles, β particles and γ particles.