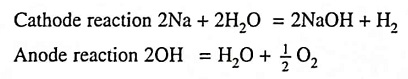Production of Chemicals by Electrolysis:
There are various industrial applications of Production of Chemicals by Electrolysis such as production of caustic soda, chlorine, potassium permanganate, ammonium per sulphate, hydrogen and oxygen by electrolysis.
The most important processes of production caustic soda by electrolysis of brine and production of hydrogen and oxygen gases by electrolysis of water are explained below.
1. Production of Caustic Soda
The oldest process is Diaphragm process. There are a number of variations, but all essentially consist of an anode compartment separated from a cathode compartment by a porous diaphragm which prevents the mechanical mixing of the two solutions. Chlorine is formed at the anode, and most of it is evolved as a gas, a small part going into solution. Sodium is discharged at the cathode and reacts with the hydroxyl ions to form sodium hydroxide and hydrogen gas is liberated at the cathode. Usually, the brine is fed into the anode compartment to resist the flow of hydroxyl ions towards the anode.
Another method of production of caustic soda by electrolytic process is mercury-cathode process. Mercury cells may be built in small (1,000 A) to very large sizes (3,000 A) or even as high as 50,000 A or more per unit. Purified saturated brine is fed to the cells and commonly circulated through them with a reduction in salt concentration. Brine is resaturated for another cycle. Each unit consists of two sections ; one which is closed and has a chlorine outlet, graphite anodes, and a mercury cathode; and a second, the denuder section, containing a mercury amalgam anode and iron cathodes. Circulation of mercury between the two sections constitutes the electrical connection. The current efficiencies depend upon the concentration of the amalgam formed in the first section and the effectiveness of the circulation of the amalgam. Current densities in mercury cells are considerably higher than in diaphragm units, being of the order of 1.5 mA or more per square mrn of anode surface.
Energy efficiencies are of the order of 50-60%. Anode consumption, when graphite anodes are employed, is of the order of 3-3.5 kg., per tone of chlorine. The voltage requirement is about 4 volts per cell.
Other production of chemicals by electrolysis, that can be obtained from electrolysis of brine by interaction of the cathodic and the anodic products are sodium hydrochloride, bleaching powder and hydrogen when the solution is cold and sodium chlorate (NaCIO3) and hydrogen when the solution is hot.
2. Production of Hydrogen and Oxygen Gas By Electrolysis of Water
Gas obtained by this process are of high purity and at a cheap cost because of the low energy consumption. The electrolyte consists of 15-20% solution of caustic soda or its equivalent caustic potash, and electrodes are of iron. Sulphuric acid is no longer used. With caustic soda as electrolyte the chemical reactions are :
Thus hydrogen and oxygen gases are liberated at cathode and anode respectively and water disappears while the quantity of caustic soda remains constant. It is, therefore, necessary to add water to the solution periodically.
The voltage requirement is 2-2.2 V per cell during operation and 2.3-2.5 V per cell during starting period. Energy consumption is about 6 kWh per cubic metre of hydrogen and 1/2 cubic metre of oxygen.
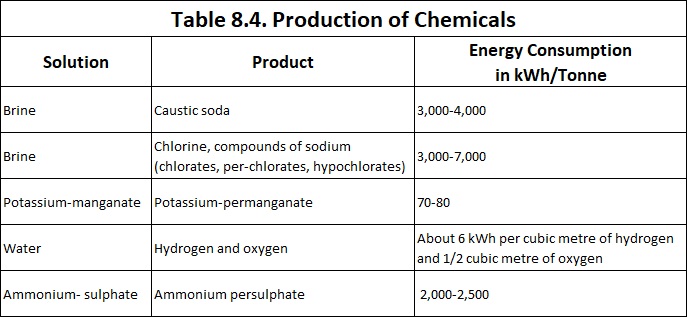
Various industrial applications of production of chemicals by electrolysis are summarized is Table 8.4.