Refining of Metals by Electrolysis:
Refining is the process whereby a highly concentrated mixture of metals is subjected to electrochemical treatment for recovering not only the principal metal in pure form, but also the precious metals like gold, silver, bismuth etc., which may be present in the form of minute traces. Some of the refining of metals by electrolysis processes are explained below.
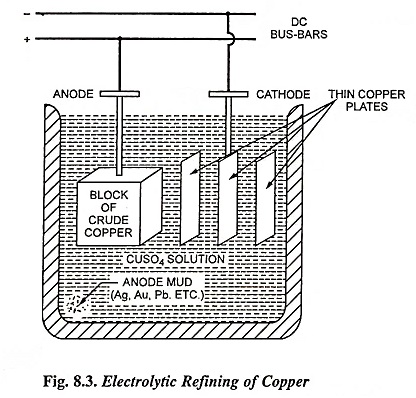
1. Refining of Copper
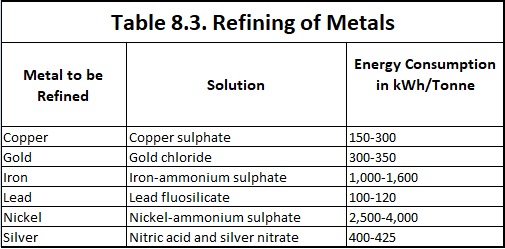
The impure copper is cast into plates which are made the anodes in a bath of copper sulphate acidified with sulphuric acid. The cathodes consist of sheets of pure copper which are coated with graphite so that the electrolyte deposit of copper may be easily stripped off. When the current is passed, copper ions are discharged at the cathode and are deposited as pure copper while hydroxyl ions discharged at the anode bring an equivalent amount of cupric ions into the solution. Thus there is a transfer of pure copper from the anode to the cathode while the impurities like nickel, zinc and iron, pass into the solution as sulphates and impurities like silver and gold, which are not affected by sulphuric acid—copper sulphate solution settles down as the anode sludge or mud. At regular intervals the deposit is stripped from the cathode and the anode is replaced. Copper extracted from its ore, known as blister copper, is 98 to 99 per cent pure. Copper of purity as high as 99.95% is obtained from blister copper by electrolytic process. Energy consumption in copper refining by electrolytic process is 150-300 kWh/tonne of refined copper.
2. Refining of Gold
Blocks of impure gold are made the anodes in an electrolytic cell containing a solution of gold chloride (AuCI3) in hydrochloric acid. Thin plates of pure gold constitute the cathodes. On electrolysis pure gold is deposited on the cathode. Gold thus obtained is 99.9% pure. For a higher purity, if desired, the metal may be put to a second electrolytic refining. The energy consumption is 300-350 kWh/tonne of refined gold.
3. Refining of Silver
Solution of nitric acid and silver nitrate is required for refining of silver with electric energy consumption of 400-425 kWh/tonne of refined silver. The refining of metals by electrolysis process is similar to that for refining of copper.
4. Refining of Nickel
The matter is first roasted and treated with dilute sulphuric acid at 80°C to remove copper. The residue consisting mostly of nickel compounds is made the anode in a solution of nickel ammonium sulphate. Iron plates are made the cathode. On electrolysis nickel deposits on these plates. The energy consumption is 2,500-4,000 kWh/tonne of refined nickel.
5. Refining of Lead
The desilverised lead can be purified further by electrolysing a solution of lead fluorosilicate (PbSiF6) and hydrofluorosilicic acid (H2SiF6) containing a little gelatin. The desilverised lead is cast into anodes while the cathodes are sheets of pure lead. Gelatin facilitates the formation of a coherent deposit of lead at the cathode. The metallic impurities which are more electropositive than lead, such as iron and tin, go into solution while the rest of the impurities are thrown down as anode mud. The energy consumption is 100-120 kWh per tonne of refined lead.
6. Refining of Zinc
The crude ore is roasted and leached with dilute sulphuric acid. The resulting solution is purified from elements present such as copper, cadmium, silver, cobalt, antimony, arsenic by treatment with the powdered zinc and with ferric hydroxide. The solution of purified zinc sulphate is then electrolysed in large concrete tank lined with lead or with rubber, using chemically pure lead as anode and aluminium cathode. The electrolysis is carried at 35-40°C using a current density of 300-400 A/m2 and a cell voltage of 3.25-3.5 volts. The zinc is stripped from the aluminium cathodes and then melted down to give a material of high purity (99.99 per cent zinc).
7. Refining of Iron
In case of purification of iron, the iron-ammonium sulphate is used as electrolyte and energy consumption 1,000-1,600 kWh per tonne of refined iron.
The summary of the some of the important refining of metals by electrolysis processes is given in Table 8.3.