What is Atomic Bonds and Types?
Whether a material is a conductor, a semiconductor, or an insulator depends largely upon what happens to the outer-shell electrons when the atoms bond themselves together to form a solid. Atomic bonds arc of two types viz. primary (or chemical) bonds and secondary (or molecular) bonds. Primary bonds are strongest bonds between atoms and can be further subdivided into (i) metallic (ii) covalent and (iii) ionic bonds. Secondary or molecular bonds are weaker than primary bonds. Attraction forces (also called winder Waal ‘s forces) exist between atoms or molecules.
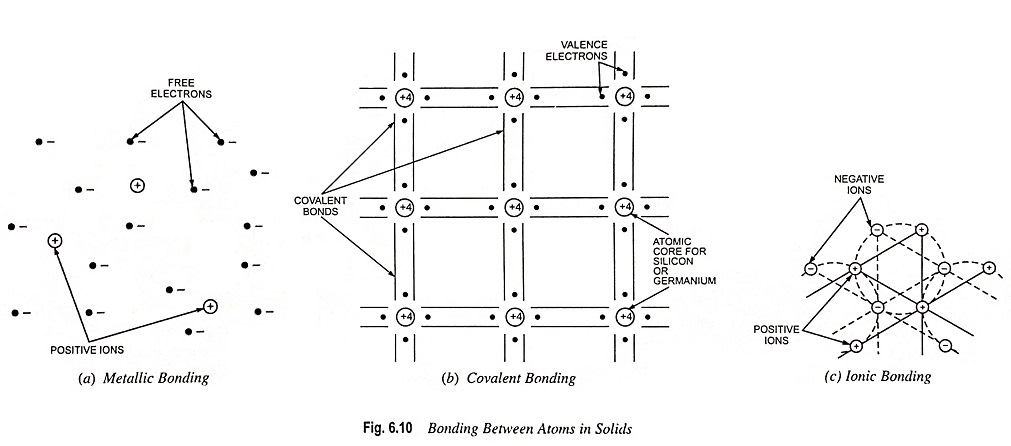
In metallic bonds, atoms give up their valence electrons to form clouds of electrons which is shared by all atoms. Such a bond exists between elements having loosely held valence electrons so that they can be easily released to the common pool.
For example, in the case of copper, the atoms give up easily detachable electrons and this creates a large mass of free electrons (or electron gas) drifting about through the spaces between the copper atoms. Each atom of copper becomes a positive ion because of losing an electron. The electron gas is, of course, negatively charged; consequently, an electrostatic force of attraction exists between the positive ions and the electron gas. This is the bonding force that holds the material together in a solid. In the case of copper and other metals, the bonding force is termed metallic bonding or sometimes electron gas bonding. Such a bonding is shown in Fig. 6.10 (a).
Covalent bond is formed by sharing of electrons by two or more atoms. It can be formed between atoms of same or different elements. This Atomic bonds arrangement is illustrated in Fig. 6.10 (b). In covalent bonding every valence shell of every atom appears to be filled, and consequently there are no holes and no free electrons drifting about within the material. The same covalent bonding arrangement exists in silicon and germanium.
The Si (or Ge) atom at the centre share one of its valence electrons with each of the valence electrons of the four Si (or Ge) atoms surrounding it, as shown in Fig. 6.10 (b). This sharing of valence electrons produces four pairs of electrons that are covalently bonded thus completing its octet.
In ionic bonds, atoms of one element transfer electrons to the other so that both have stable configurations.
In this arrangement, some atoms part with outer-shell electrons, but these are accepted into the orbit of other atoms. Thus, the atoms are ionized; those which give up electrons become positive ions, and those which accept the electrons become negative ions. This creates an electrostatic bonding force between the atoms, called the ionic bonding [Fig. 6.10 (c)]. Such bonding occurs in some of the insulating materials.