What is Electrolytic Process and Basic Principle of Electrolysis
The fact that electrical energy can produce chemical changes and the processes based on it, called the ‘electrolytic process’, are widely used for the extraction of pure metals from their ores (such as aluminium, zinc, copper, magnesium, sodium etc.), manufacturing of various chemicals (such as caustic soda, potassium permanganate, hydrogen, oxygen, chlorine etc.), electrodeposition of metals including electroplating, electrotyping, electroforming, building up of worn-out parts in metallurgical, chemical and other industries. Though the various processes mentioned are different in apparent detail but fundamentally they are alike, being based on the principle of electrolysis.
Basic Principle of Electrolysis
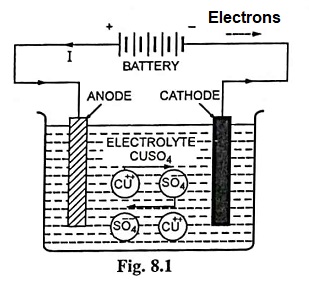
In nature atoms of electrolyte (chemical compound) are closely bound together but bond becomes weaker, when dissolved and the molecules of the electrolyte split up into two types of ions carrying electric charges, called the cations and anions, and moving freely in the solution. Now if two electrodes are dipped into the electrolyte and connected to the dc supply, ions associated with positive charge (cations) and moving freely in the solution are attracted by the cathode (electrode connected to the negative terminal of the supply) and the ions associated with negative charge (anions) and moving freely in the solution are attracted by the anode (electrode connected to the positive terminal of the supply).
For example, when copper sulphate (CuSO4) is dissolved in water, immediately it gets dissociated into +vely charged copper ions (Cu++) and negatively charged sulphions (S0––4) moving freely in the solution and if a potential difference is applied between the two electrodes immersed in the solution the +vely charged copper ions will move towards the cathode and the –vely charged sulphions will move towards the anode. Each of the positively charged copper ions reaching the cathode will take two electrons from it and become a metallic atom of copper, and similarly each of the negatively charged sulphions reaching the anode will give up two electrons to it and cease to be anion. Thus the electrons will move from anode to cathode in the external circuit and constitute flow of current from anode to cathode in the electrolyte. Thus the function of source of supply seems only to serve as an electron pump pumping electrons from +ve side of the supply and supply to the –ve side of the supply.
As mentioned above each +vely charged copper ion on reaching the cathode takes two electrons from it, becomes atom of metallic copper and deposite there. Similarly each –vely charged sulphion on reaching the anode gives up two electrons to it and becomes SO4 radical but since SO4 radical cannot exist in the electrical neutral state so it will attack the anode and will form the corresponding sulphate of the material of the anode; for example if the anode is of copper then copper sulphate (CuSO4) will be formed, but if the anode is of such a material which cannot be attacked by SO4, for example if the anode is of carbon the SO4 will react with water and form sulphuric acid and liberate oxygen according to chemical reaction
2SO4 + 2H2O → 2H2SO4 + O2
The whole electrolytic process described above is called the electrolysis and the effect is that the copper gets dissolved from the anode and deposited on the cathode. During the process there is no accumulation of charge at any point in the circuit and the mass of copper deposited at the cathode is exactly equal to that removed from the anode.